Adult Hippocampal Neurogenesis
Dec 26, 2020 | 3 min read
Dec 26, 2020 | 3 min read

Neurogenesis is the process of formation of new neurons in the brain and is a very crucial aspect of embryonic development. While the topic of adult neurogenesis in humans has been a controversial one since it was first proposed by Eriksson et al.
in 1998, many studies have since shown that some regions of the brain do in-fact, retain the ability to generate new neurons throughout the lifespan of the organism.
The hippocampus is a small but incredibly complex structure that is embedded deep inside the temporal lobes of the human brain. It is one of the components of the limbic system and its primary functions include the formation of new declarative memories
along with spatial processing and navigation. Being one of the most extensively studied structures in the brain, one of its notable features was found to be its high susceptibility to plasticity. The Dentate Gyrus (DG) is a part of the hippocampus that
has been implicated in the formation of new episodic memories, which are unique memories of an individual for a specific event.
Adult hippocampal neurogenesis has been well demonstrated in many mammalian species including rodents and is believed to play an important role in aiding learning and memory. Although these new-born neurons develop slowly, they have been known to have increased plasticity
and are supposedly important for refining and modulating existing neuronal circuits as well as provide a neural substrate for the accommodation of new experiences. They are also believed to play a role in the prevention of neurodegeneration.
Findings:
In this article, an attempt was made to find evidence of Adult Hippocampal Neurogenesis (AHN) in aging human brains by looking for populations of immature neurons in the adult DG that persist throughout life. They found neurons in varying stages of maturation
in the adult DG, with a declining rate of AHN during physiological aging in humans.
Human brain samples of the DG region were analyzed for DCX+ cells, initially in neurologically healthy subjects. Doublecortin (DCX) is an essential factor for neurogenesis and is expressed by neurons in most of the differentiation stages of AHN in rodents. When
DCX+ cells were analyzed in humans, they found a population of cells in varying stages of maturity with a subset of the population positive for markers that are expressed by immature neurons like calretinin.
In addition to this, to confirm that they had actually found neurons in varying degrees of maturation, they analyzed populations of double-labeled cells (DCX+ CR+ and DCX+ CB+) and observed their morphological heterogeneity. Calretinin (CR), as mentioned, is a
calcium-binding protein that is transiently expressed by immature neurons whereas Calbindin (CB) is more characteristic of mature neurons. In keeping with expectations, they found that cells that were doubly positive for DCX and CR displayed an immature neuron
phenotype while those that were doubly positive for DCX and CB had more of a mature neuron phenotype.
Thus, they arrived at the conclusion, considering the percentages of doubly positive cells representing immature and mature neurons, that adult hippocampal neurogenesis persists in the organism, well until the 9th decade of life (the oldest brain tissue sample
studied was from an 87-year-old patient).
Another interesting aspect of the study was focused more on the rate of AHN in patients that were suffering from Alzheimer’s disease. They had evidence that the population of DCX+ cells reduced parallelly with age in neurologically healthy subjects. However, in
Alzheimer’s, they found that the rate of decline of AHN was much faster as the number and maturation of these neurons decreased as the disease progressed. Thus, some other mechanisms might be at play that destroys the immature neuron population in Alzheimer’s
disease setting it apart from normal aging. Furthermore, these changes in AHN were detectable at the early stages of the disease and also supposedly years before clinical symptoms occur.
Thus, they suggest using AHN impairments as a biomarker for early detection of Alzheimer’s disease using non-invasive methods. Moreover, improving the numbers and functionality of immature neurons in DG may be an important therapeutic strategy that could be implemented
to slow down or prevent Alzheimer’s disease progression.
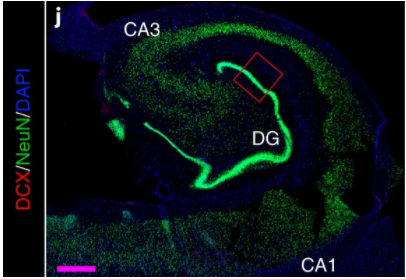
Figure 1. This is a microscopy image of whole adult human hippocampus wherein DG stands for the Dentate gyrus region. The different markers have been shown in different colors to find subpopulations of neurons in the hippocampus. Source