Brain-gut-microbe Communication in Health and Disease
Dec 30, 2020 | 1 min read
Dec 30, 2020 | 1 min read

The gastrointestinal tract(GIT) and brain communicate with each other on various levels - neural, hormonal, and immunological, essential for maintaining homeostasis, a normal body state. This bidirectional communication system is known as the brain-gut-enteric microbiota axis. Microbiota refers to the microorganisms present in the GIT.
This article discusses the critical components of this axis, and the methodological challenges while determining what constitutes a normal microbiota and charting its development over time. New insight on how these microbiota may affect mood and behaviour is explored, along with possible mechanisms. Therapeutic opportunities for treating central nervous system (CNS) disorders by targeting the microbiome are critically analysed.
Like any healthy relationship, the brain and GIT have a two-way communication network. Signals from the brain can influence the motor and secretory workings of the GIT, and messages from the GIT can influence brain function. The inhabitants of the microbiota range from 1013-1014 microorganisms, which is 150 times as many genes in the human genome! Rest assured, there isn’t a lack of diversity, with more than 7000 strains in the adult microbiome. It largely consists of anaerobic bacteria, although viruses, protozoa, archaea, and fungi are also present.
Though these descriptions sound impressive, they might not be all that accurate. The standard culture-based analyses completely ignore the gut microbiota that are less than amenable to cultivation. To compensate for this loss, there are culture independent techniques employed, like quantitative PCR, FISH, and genetic fingerprinting. The culture technology has not caught up to these alternative methods. This results in a considerable logistic challenge, as two sets of techniques need to be conducted for a somewhat complete analysis.
The human body is no stranger to colonisation. The infant gut, during delivery, is exposed to a multitude of microbes, and the initial microbiome has a strong maternal influence. Over time, like the rest of the infant, the microbiome too learns and grows, expanding in number and diversity.
To study how the microbiome changes, germ-free(GF) animals are used. They have a sterile gut environment, as they are surgically delivered, which removes the possibility of post-natal colonisation of the GIT. However, they do not reflect real life scenarios. Broad spectrum antibiotics are used to intentionally alter the microbiome in a reproducible manner.
Does the microbiota have an effect on the immune system?
Most definitely. Through germ-free studies, it has been found that the secretion of IgA (Immunoglobulin A) and controlled inflammation are a consequence of bacterial colonisation. GF animals, once reacquainted with the intestinal flora, are seen to develop the mucosal immune system. Preventing pathogens from colonising is achieved by competition for nutrients and production of ant-microbial compounds. Lactobacillus and Bifidobacterium were shown to inhibit listerial infections, the former by acid production and protein secretion, and the latter by an extracellular proteinaceous compound.
Perhaps the most surprising effect of the microbiota is on mood and behaviour. The most consistent data has been in relation to anxiety. Chronic treatment of rats with L. rhamnosus, a probiotic, results in lower levels of the stress-induced corticosterone and a less anxious phenotype in the elevated plus maze.
There are various mechanisms of action proposed for the communication network, the most convincing of those involving the vagus nerve, also called the pneumogastric nerve.
Since the microbiota is involved in wellbeing, it certainly has a role to play in disease. Obesity and irritable bowel syndrome(IBS) have strong connections to irregular gut functioning. However, less intuitive is their potential role in the development of autism, which is explained in the paper.
Lastly, the interaction of the microbiota and CNS implies therapeutic potential for CNS disorders. This field is still developing, and no conclusive discoveries have been made yet, but a recent study shows positive psychological effects in healthy human volunteers administered with a combination of probiotics. (L. helveticus and B. longum)
This has opened up multiple possibilities for the treatment of mental health issues like anxiety and depression. Probiotics could become a range of therapy all by itself. Though there is still a long way to go, we must remember the importance of this microbiota axis, and trust our gut.
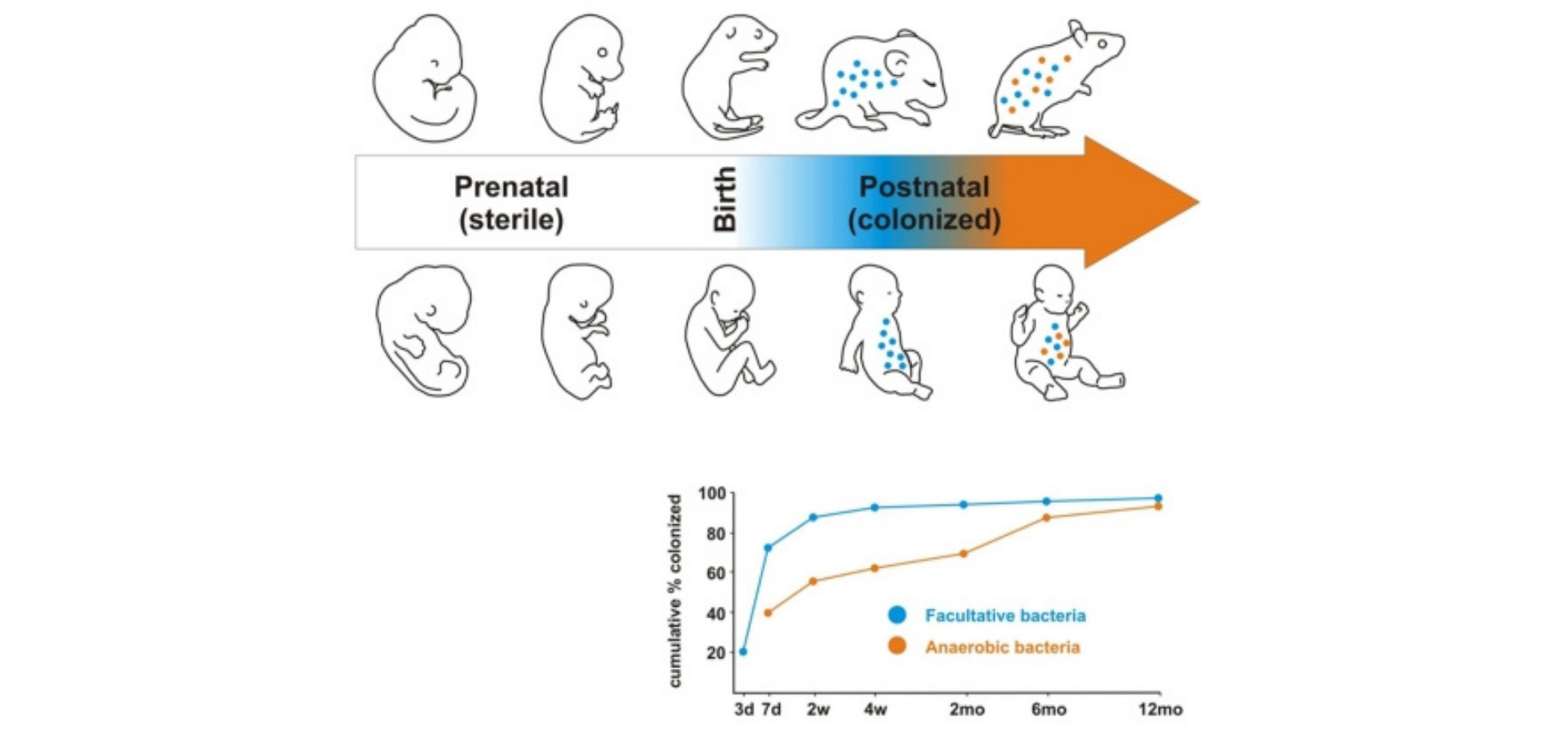
Figure 1. Development of the microbiome in early life. Subsequent to the sterile uterine environment, colonization begins at birth with facultative bacteria colonizing the GIT immediately. The anaerobic bacteria colonize later.
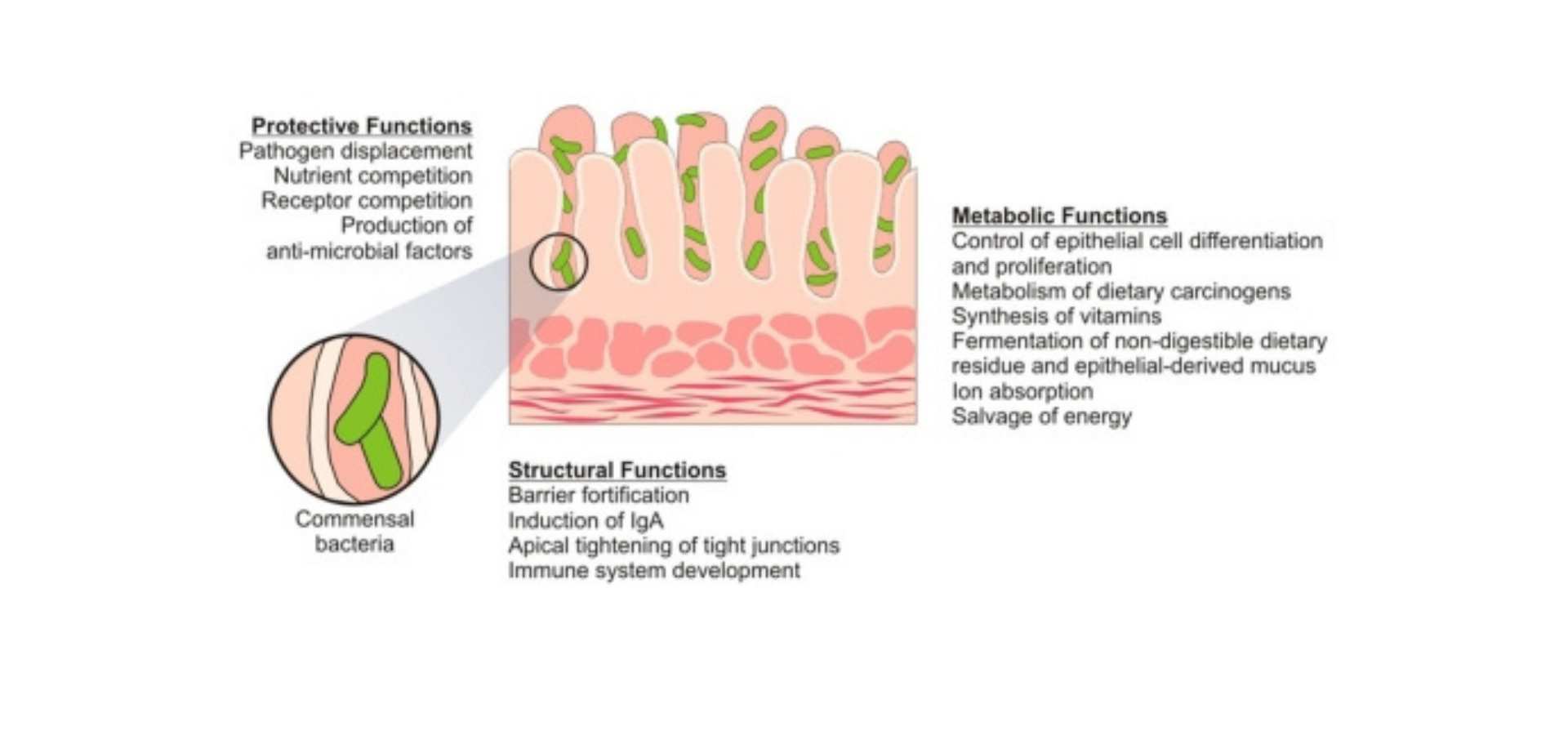
Figure 2.Function of Intestinal Microbiome. Commensal bacteria exert a miscellany of protective, structural and metabolic effects on the intestinal mucosa.
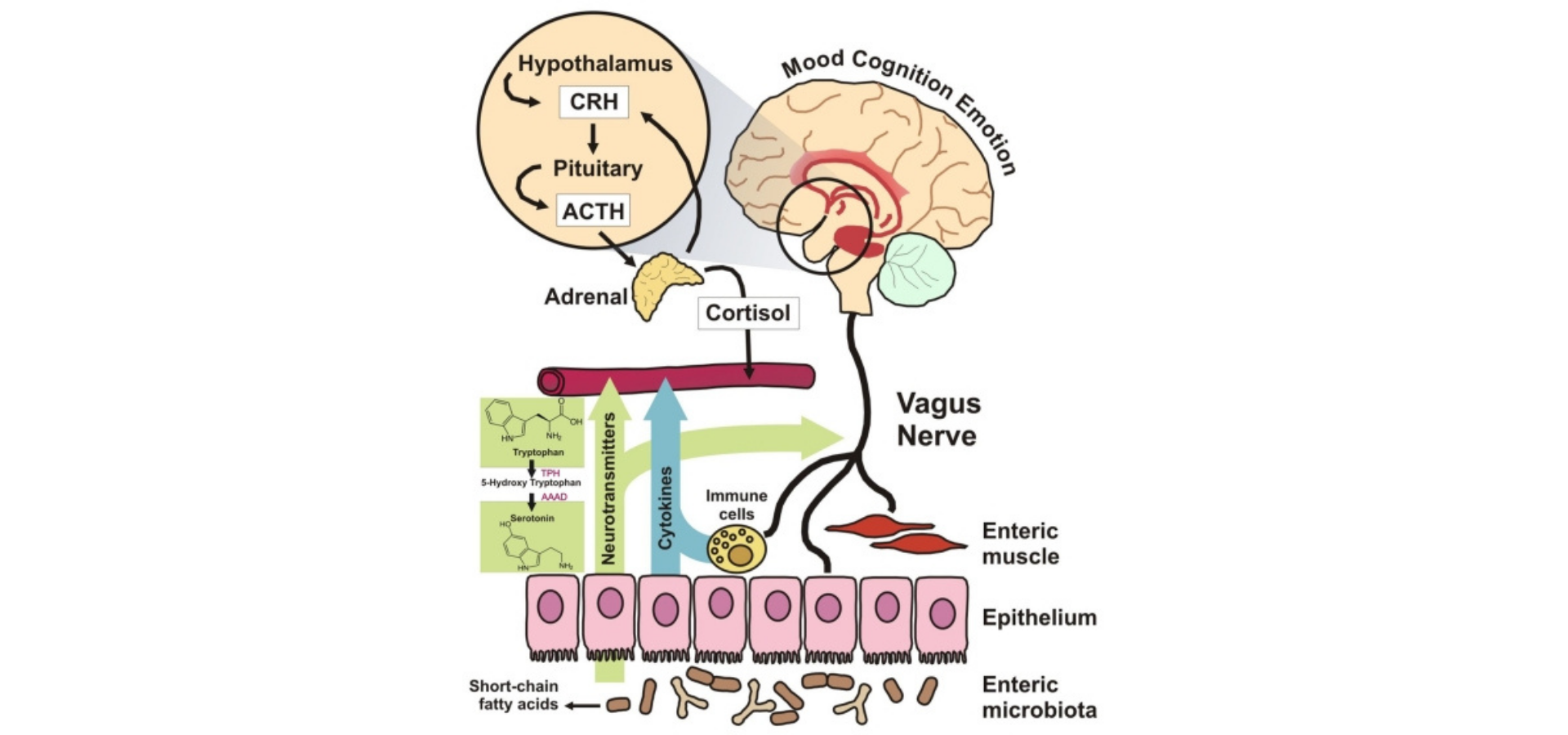
Figure 3.A variety of proposed mechanisms of the interaction between the brain and the gut microbiota.