Calvin: The Photosynthetic Cycle
July 12, 2021 | 2 min read

July 12, 2021 | 2 min read

Melvin Calvin is a renowned biochemist known for his discovery of the Calvin cycle along with Andrew Benson and James Bassham. He and his team deciphered that the photosynthetic cycle, previously known as “The path of carbon in photosynthesis” is not carried out in living materials or formed parts of living cells; but in fact in a separate collection of soluble chemicals. When coming out with this biosynthetic mechanism, he explained, “Thus, the principles of the transformations are simpler, but the actual details of the transformations are more complex.” (when compared to the findings of other organic chemists at that time.)
Experiments Carried Out:
The first experiments carried out were described as ‘Appearance-Rate Experiments’ - wherein plants were fed with radiolabeled carbon dioxide and a series of snapshots of the chemical distribution of the radiocarbon was taken as it passed through and arrived as carbohydrates. Here, an X- Ray film was placed with the paper and where a radioactive spot was identified, the X- Ray film was exposed which would provide a series of dark spots for particular compounds of interest with radiolabeled carbon. To identify the chemical structure, the migration of the compounds were analyzed initially. This was followed by cutting out the sections of the film and eluting the compound in a suitable solvent. On this, various chemical operations were done before running it in a two-dimensional chromatography. The behaviour seen here after different chemical treatments allowed for narrowing of the chemical characteristics of the compound. Final identification was done by mixing the radiocompound with the supposed chemical it is and conducting a colour test.
By breaking the acid apart by chemical methods, they were able to decipher which carbon was which in a separated form and further, the distribution of radioactivity in the individual carbons was found.
Example:
In PGA (3 - phosphoglyceric acid), most of the radioactivity was found in the carboxyl group and the other two carbons were equally radioactive. Similarly, in hexose, they found that the radioactivity of the two middle carbons was such that they were formed from the carboxy carbon, and the outer four corresponded to the other two carbons of PGA. From this, it was initially thought to be the reverse of glycolytic fission.
To determine the origin of PGA, the task met by the researchers was to find a two carbon compound that accepts carbon dioxide. This took many years. In the process, compounds such as ribulose, sedoheptulose, and their mono and di phosphates were found. Similar checks for the radioactivity of individual carbons indicated that in ribulose, the radioactivity of the third carbon was the most, followed by carbon atoms 1 and 2, and finally carbon atoms 4 and 5. In the sedoheptulose, the three center carbon atoms (3,4,5) had the most radioactivity followed by carbon atoms 1 and 2, then 6 and 7. Extremely short experiments, of the order of milliseconds, did show a low value for the fourth carbon atom in sedoheptulose.
The peculiarities of the radioactivity of C7 is what makes the determination of its formation difficult. Was it formed by a C5 + C2 reaction or a C4 + C3 reaction?
Ans.
The nearly equal distribution of the center three indicate that there is no intact element of the five-carbon ribulose present in the sedoheptulose, since there is no intact group of five carbon atoms which has the same labeling pattern as we see in the ribulose. So C5 + C2 cannot be the formation reaction.
Consider the C4 + C3 method for the construction of sedoheptulose. The availability of the C3 fragments is clear enough for the phosphoglyceric acid. The hexose seems, therefore, a possible source of the C4 fragment which, when combined with a fragment directly related to PGA, will give rise to a sugar, sedoheptulose.
This can be done by taking a four-carbon sugar made of carbon atoms 3, 4, 5, and 6 (that is, the lower four carbon atoms) of fructose, in which the first two (3 and 4) would have the label, and condensing it in an aldol type condensation with phosphodihydroxyacetone. If the combined sizes of the tetrose and triose are very small, it should be possible to arrive very quickly at a heptose in which the center three carbon atoms are very nearly equally labeled.
Pentose can be made by removing a carbon atom from the hexose, or by building it up from smaller fragments, i.e., by adding a C1 to a C4 fragment, or by adding a C2 to a C3 fragment. The lack of relationship between the pentose labeling and any five-carbon sequence in the hexose allows the elimination of the construction of the pentose by loss of a terminal carbon in the hexose.
Removing the two-carbon fragment from the top of the sedoheptulose we could make two five-carbon compounds which, taken together with the five-carbon compound already formed, would produce the labeling scheme finally observed in ribulose diphosphate.
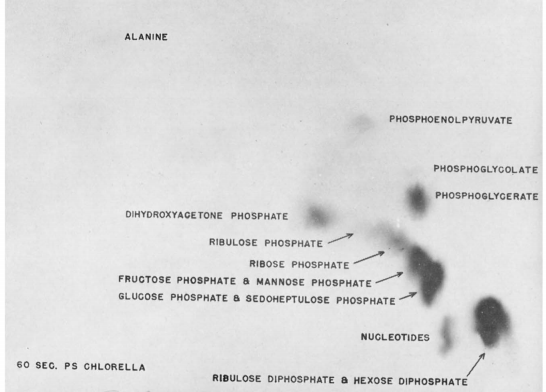
Fig 1.
Chromatogram of extract from alga indicating uptake of radiocarbon during photosynthesis with 60s exposure in Chlorella.
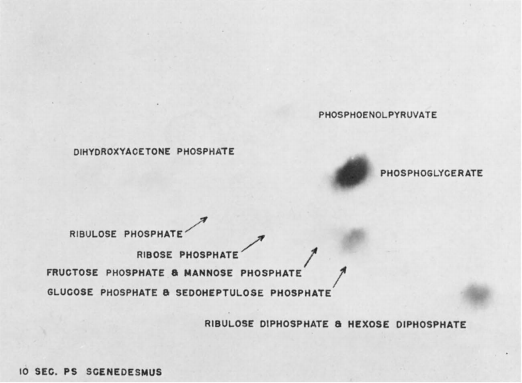
Fig 2.
Chromatogram of extract from alga indicating uptake of radiocarbon during photosynthesis with 10s exposure in Scenedesmus.
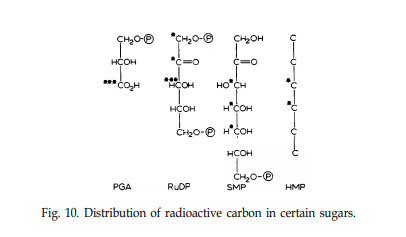
Fig 3.
The peculiarities of the radioactivity of C7 is what makes the determination of its formation difficult. Was it formed by a C5 + C2 reaction or a C4 + C3 reaction?