Differential Scanning Calorimetry
Aug 11, 2021 | 4 min read
Aug 11, 2021 | 4 min read

Differential scanning calorimetry is a thermoanalytical technique used to study the thermodynamic properties of biomolecules. This method measures the amount of heat energy transferred from or to a constant pressure sample during a physical or chemical change.
The apparatus consists of two compartments: one for the sample material and the other for the reference material. Both compartments are heated up at a uniform rate. The temperature of both the materials would be the same initially. However, if a physical or chemical reaction occurs in the sample material, its temperature would vary considerably from that of the reference material. Heat energy is transferred to or from a sample for equalizing the temperature of both materials. This heat energy transfer is attributed to the variation of the sample's heat capacity at constant pressure (Cp), as the temperature varies.
Using the differential scanning calorimeter (DSC), one can plot a thermogram- a graph between Cp and temperature. The thermogram is a great tool to analyze the thermal stability of proteins, membranes, nucleic acids, and other biomolecules.
One significant advantage of the DSC is that the analysis can be done using samples of masses as low as 0.5 mg, while other calorimeters require at least a few grams of the sample. Also, the thermodynamic data obtained from the DSC is sensitive to the biomolecule's structure. The thermogram changes according to the changes in the structure of the biomolecule.
In the case of proteins, the Cp change could be related to the disruption of various forces that stabilize the protein structure, such as hydrogen bonds, hydrophobic and electrostatic interactions, etc. A temperature change denatures the protein- a loss of the three-dimensional structure and the biological activity. The temperature corresponding to the peak in the thermogram is called the transition midpoint (Tm). At this temperature, the native structure of 50% of the proteins is intact, while that of the other 50% is lost. A protein with a higher Tm value possesses more stability than a protein with a lower Tm value. So this technique could be used to identify proteins with greater stability and the conditions that should be maintained to ensure the protein's stability.
When membrane lipids that are insoluble in water come into contact with water, the spontaneous formation of complex structures occurs. These structures are explained in terms of lipid phases. The phases generally observed in lipid-water systems are hexagonal, cubic, lamellar, micellar, and monolayers.
In the pure form, most membrane lipids are found in the lamellar phase, which is further subdivided into five sub-phases. Phase transitions due to change in temperature are called thermotropic transitions. DSC can detect such thermotropic transitions, such as the interconversions among the various lamellar phases and the transition of the lamellar to a specific hexagonal phase.
For the efficient delivery of the recombinant DNA into the cell, DNA is often represented in complexes with cationic lipids (lipoplexes). Hydration, an important criterion of lipoplexes, has been measured by. D. Hirsch-Lerner and Y. Barenholz using DSC.
Peptide nucleic acids (PNAs) are synthetic analogs to DNA. The backbone of PNA is not charged, unlike that of DNA. Hence PNA-DNA and PNA-RNA duplexes possess higher thermal stability than DNA-DNA and DNA-RNA duplexes. DSC can be very effectively used in the study of the thermal stability of PNA-DNA and DNA-DNA duplexes.
There are also instances where DSC was used to analyze carbohydrates. Gelatinization is a process in which starch granules undergo a physicochemical transformation when subjected to heat treatment in a solvent's presence. Gelatinization can lead to many changes in the starch granules, such as swelling, improved solubility, and digestibility, amylose exudation, etc. DSC appears to be the most practical technique for the analysis of the gelatinization process.
The platelet cells in the blood are responsible for the formation of clots that stops bleeding. A constraint in the storage of platelets is that they are irreversibly activated at 4 degrees Celsius. It is an after-effect of platelet cells' thermotropic transition from fluid to gel state at 15 degrees Celsius. The DSC technique has been used to study this thermotropic transition of platelets.
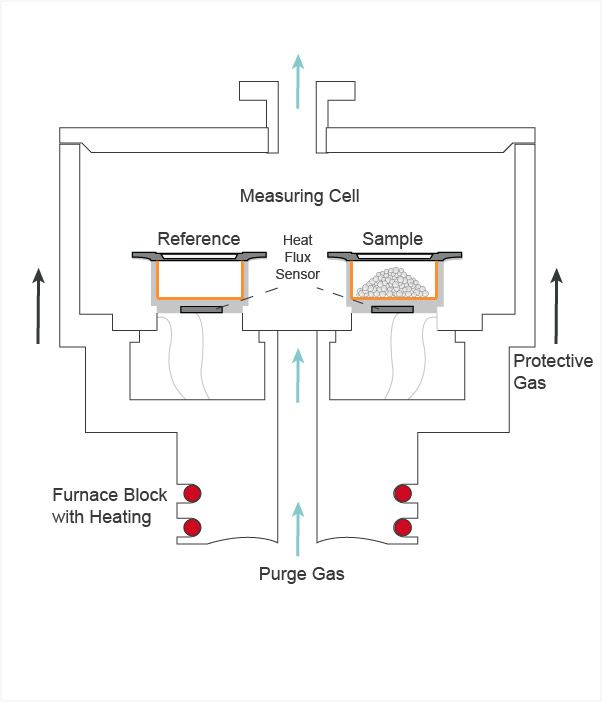
Figure 1. Differential scanning calorimeter Source
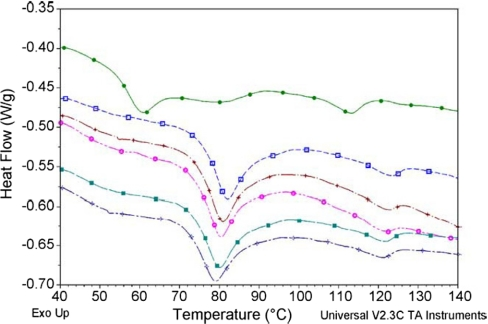
Figure 2. The gelatinization thermograms of the starch–fructose–glucose mixtures. Source