The Discovery of mRNA Splicing
October 21, 2021 | 6 min read
October 21, 2021 | 6 min read

Throughout the course of education and training in the biological sciences, we are introduced to a wide array of concepts, techniques and discoveries, which often reflect the life’s work of a scientist/group of scientists. It is important to go back from time to time and appreciate the ideas and work that went into the discoveries we so casually glance through in textbooks and presentations.
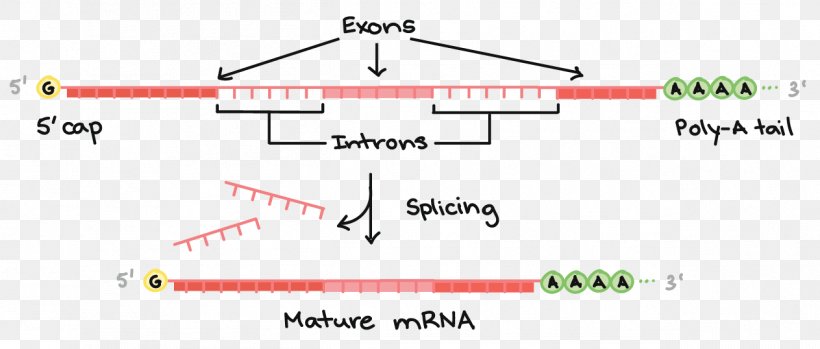
We all know that mRNA splicing is a fundamental part of RNA processing in most eukaryotic and some prokaryotic genes. The idea is simple; you take a pre-mRNA (precursor mRNA) and splice out (remove) some intervening sequences called introns to get mature mRNA (of course, capping and polyadenylation also occur). Splicing is useful for the cell to generate different protein variants from the same gene transcript; this process is referred to as alternative splicing. For example, in the figure shown below, removing both introns generates one splice variant, and two other variants can be generated by splicing either intron and keeping the other. This is one way the number of proteins produced from a fixed amount of genes can be increased.
But how was splicing discovered? Who were the scientists responsible for the initial discovery, and what did they do? To start off, the 1993 Nobel Prize in Physiology or Medicine was awarded to Phillip Sharp and Richard Roberts for “the discovery that genes in eukaryotes are not contiguous strings but contain introns, and that the splicing of messenger RNA to delete those intons can occur in different ways, yielding different proteins from the same DNA sequence"
One of the initial papers reported from the lab of Phillip Sharp (Spliced segments at the 5’ terminus of adenovirus 2 late mRNA, Berget et al., Proc. Natl. Acad. Sci. USA, August 1977) relied on the electron micrographic study of R-loops. R-loops are simply triple helical structures which have an RNA:DNA hybrid, along with a ssDNA strand that was displaced by the RNA.
As the title of the paper suggests, the authors were studying R-loops with adenovirus 2 mRNA.
When they hybridised the mRNA with adenovirus DNA, they noticed some overhangs i.e regions where the RNA: DNA binding was not perfect, as shown in the figure, which has been taken directly from the paper.
Single stranded RNA is represented by the arrows. Such single stranded RNA is not expected if the RNA sequence is exactly complementary to the DNA, which gave the scientists a clue that perhaps some sequences present in the DNA were absent in the RNA (i.e they might have been sliced out during RNA processing). Of course, their attention to detail must be commended; often,we ignore small details that we can’t seem to explain in the lab (like the single stranded RNA tails in this case), which however might provide valuable insights if only we pursued them further.
The paper also considers alternative possibilities and eliminates them one by one. For example, one possibility is that the other strand of DNA simply displaced the RNA due to a change in conditions, and that's why you see single stranded RNA. The authors test this theory by forming RNA hybrids with single stranded DNA to eliminate this possibility. However, single stranded RNA tails/loops were still observed.
There are a few more alternate hypotheses which are considered, tested, and finally disproved, leading the authors to the preliminary conclusions that would reshape biology. We strongly recommend that you go through the paper for a better appreciation of the techniques, materials and methods: a link is given along with this article
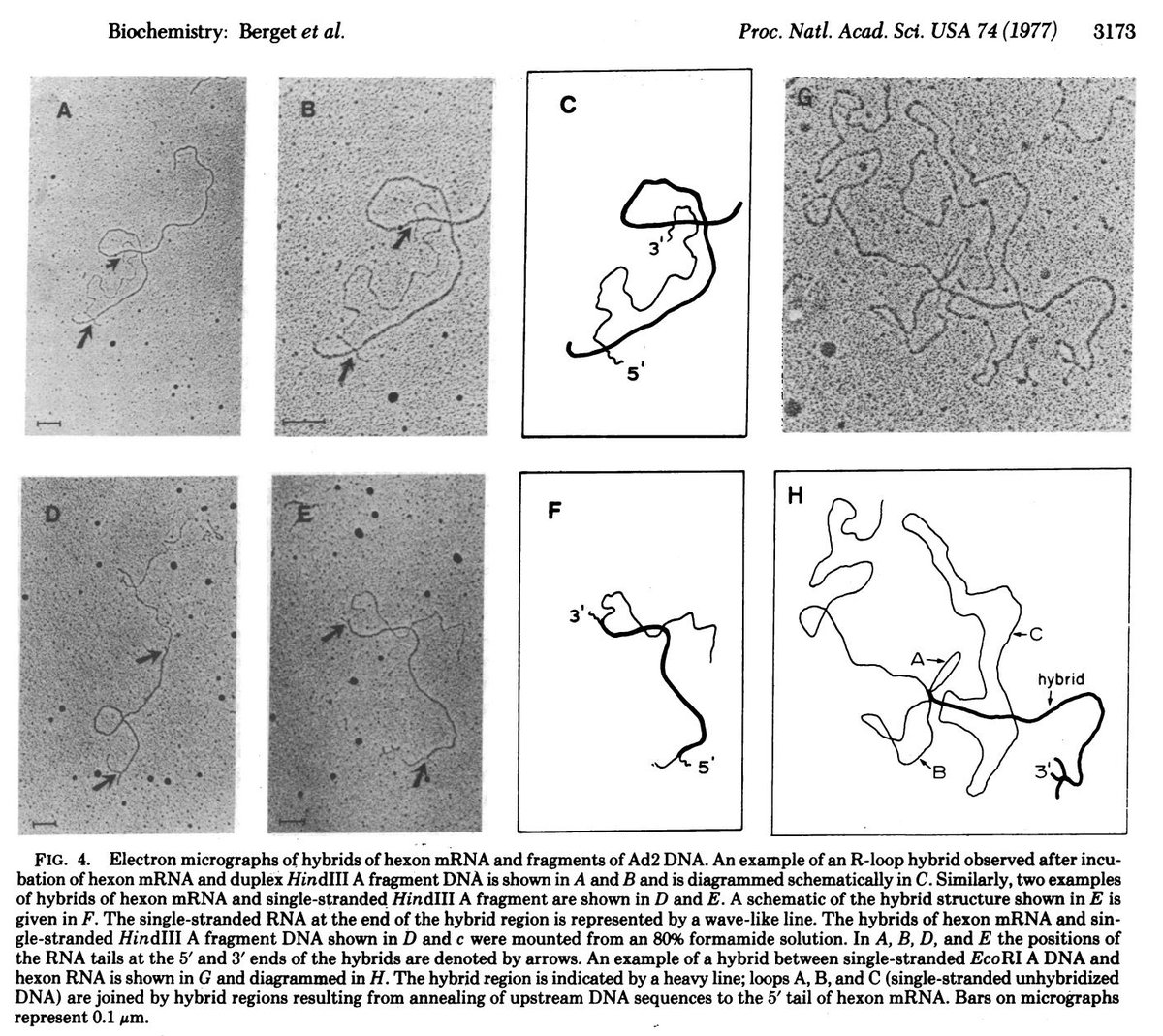
Figure 1.
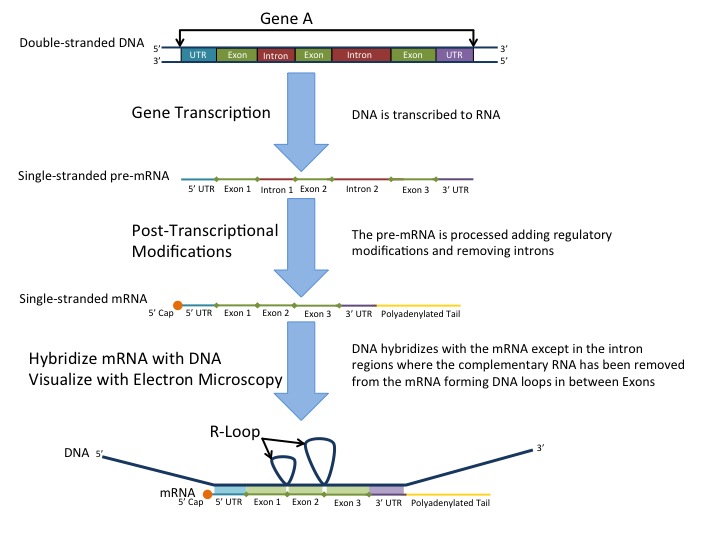
Figure 2.