Optogenetics
Oct 26, 2020 | 1 min read
Oct 26, 2020 | 1 min read

Optogenetics is an innovative approach of combining optics and genetic engineering such that it facilitates precise controlling and manipulation of individual neurons. In this technique which has revolutionized the field of neuroscience,
light is used to control neurons that have been genetically modified to express light-sensitive ion channels. In a scenario where the traditional assessment of neurons by direct stimulation of brain tissue using tiny electrodes fails to
guarantee precision, optogenetics provides millisecond scale temporal precision. In recent years, it has proved to have the potential to unveil the relation between the function of specific neurons and behavioral patterns.
The era of optogenetics started in 2002 when researchers discovered that the protein which caused the green algae to swim towards or away from light, is a light-sensitive channel, channelrhodopsin-2. Blue light caused the channel to open,
through which positive ions (Na+) flooded into the cell which was similar to the influx of ions which caused the neurons to fire. Scientists realized its potential in mammalian cells and in 2005, Karl Deisseroth's laboratory, including
graduate students Ed Boyden and Feng Zhang published the first demonstration of a single-component optogenetic system in neurons using the channelrhodopsin-2.
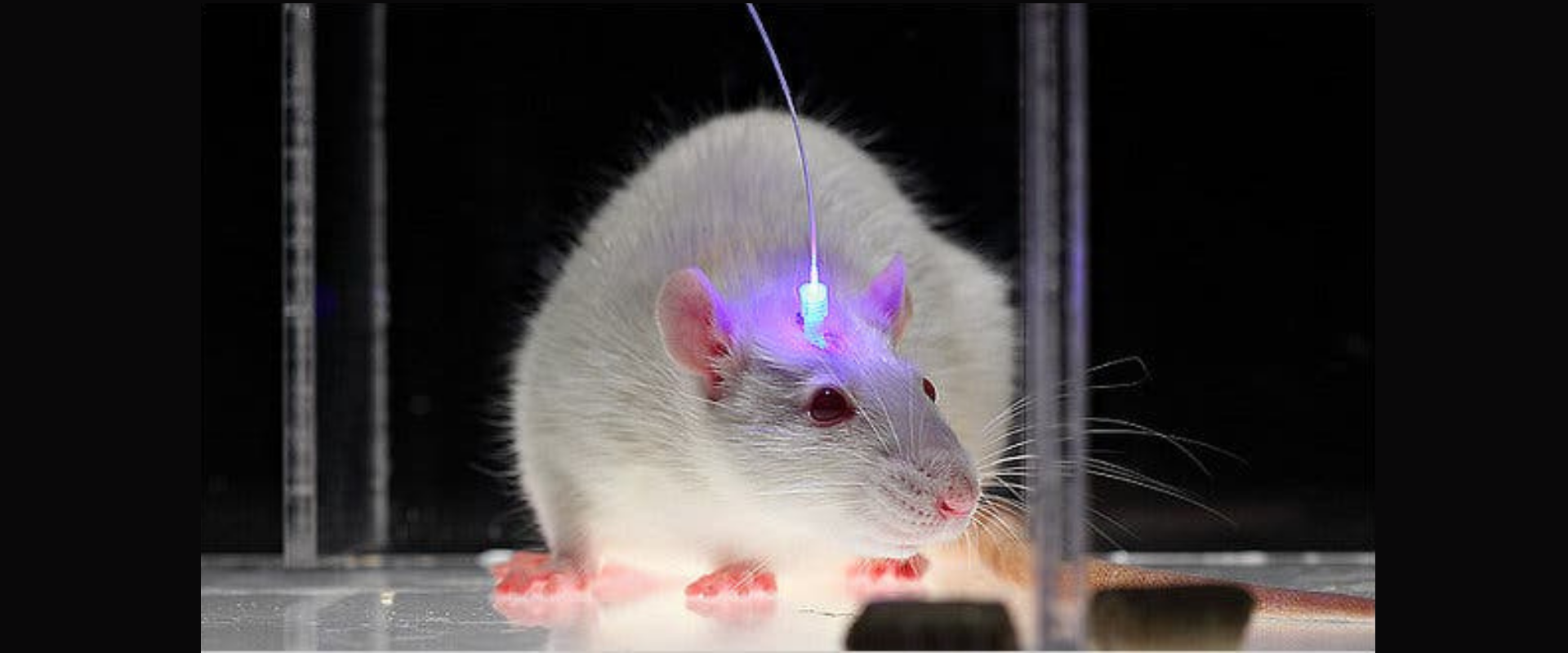
In optogenetics, the basics of genetic engineering are used to incorporate the algal protein into the animal brain. Gene therapy techniques are used to encapsulate genetic material into a viral vector. The genetic material in the virus is
designed to encode cells with a light-sensitive protein called opsin. These viruses are delivered to targeted neuronal cells via an injection that transduces the genetic sequence of opsin. Either using fiber optics or wireless implant, a
pulsing light source triggers the neuron to respond electrically and pass it to adjacent cells as a chain reaction which eventually results in a response such as muscle contraction.
With new tools allowing control over a variety of diverse cellular events, optogenetics is now poised to prove its potential in other biological fields as well. Mouse heart cells engineered to beat with pulses of light and skin cells
incorporated with light-sensitive protein moving towards laser are a few examples to count. From a clinical perspective, Optogenetics blooms as a hope in the treatment of neurological disorders, vision restoration, and deep-brain stimulation
in motor diseases. Optogenetics-driven research has already led to advancements in many neurological and psychiatric disorders including Parkinson’s disease, epilepsy, autism, anxiety, addiction, and depression.
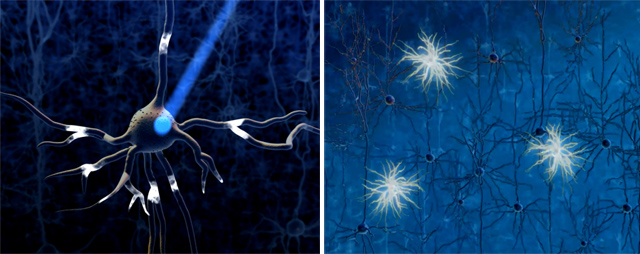
Figure 1. A technique that was chosen as the “Method of the Year “across all fields of science and engineering by the interdisciplinary research journal Nature Methods in 2010 and was emphasized by the research journal Science as the “Breakthrough of the Decade”. Source